On a quiet sunny day, my class went to Eagle Cove, San Juan Island to dig Abarenicola claparedi for our first dissection. The best and easiest way to find these worms is looking for their burrow openings and fecal castings on the surface of the sand. Then we used the shovels to dig as much sand as we could and searching them.
Abarenicola claparedi belongs to the class Polychaeta, phylum Annelida. Figure 1 and 2 shows the appearance of the A. claperedi. The whole worm is 20cm long, and1cm wide at the head. The first segments, including the head, are green, followed by yellow body segments. The color of the mouth is red and the anus is orange-red. There are red branchia on both sides of the middle region. A. claperedi lives in borrows in the sand. It swallows the sediment and feeds on the organic material. Its feces can be seen as sandy ropes outside of the opening to its burrow. Meanwhile, the sand will collapse a little because of the swallow.
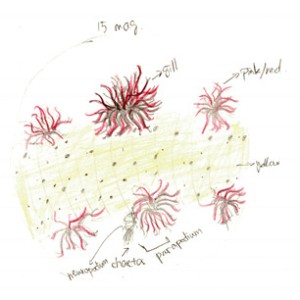
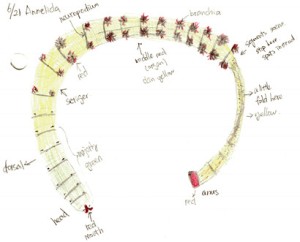
Figure 3. Drawing of dorsal view with branchia. At 15x magnification of dissecting scope. Also shows the complete part of parapodium
Looking closely to the skin, there are orange dots covering the skin. The parapodium consists of a ventral neuropodium with chaetae and a dorsal brachium (gill). Figure 3 shows the details of the branchia. Lots of branches increase the surface area for gas exchange. The red color is respiratory pigment in capillaries that go through each branch, transferring oxygen while “breathing”. Observed at 15x magnification on the dissecting scope, the skin of the A. claperedi is translucent yellow: you can see the gut but not clearly underneath the skin.
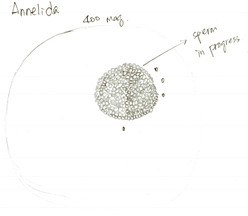
Figure 4. Drawing of a maturing spermatocyte finding in the body liquid of A. claperedi. At 400x maginification of compound scope.
Finishing observations of external features, we started to dissect the A. claperedi. We cut a small hole with fine scissors and sucked some liquid flowing out from the worm. Figure 4 shows what we saw under the compound scope. We didn’t see mature gametes, but think that the cell in figure 4 is a maturing spermatocyte. The season that A. claperedi spawn sperms is spring and we dissected it in summer.
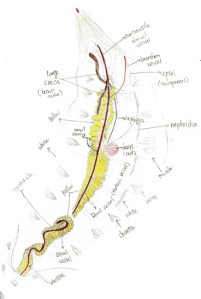
Figure 5. Drawing of the opening body of A. claperedi after dissection. Anterior up, posterior down. Not the compelet worm with part of bottom left. Shows all the organs and muscles. Differenciated by color pencle.
Figure 5 shows the internal structure of A. claperedi. The organs are clear and organized inside its coelom. From the posterior, you can see the intestine, with black sediment in it. It is connected with the stomach. Our worm’s stomach broke in to two parts, maybe in the process of digging it out from the sediment. Anterior to the stomach, you can see the esophageal caeca. There are around 10 to 13 small caeca arranged along the esophagus, posterior to two long, large caeca. Both the stomach and the small caeca are yellow and the large caeca are brown. Between the stomach and the caeca is the heart, in dark red. On the surface of that organ, a relative thick dorsal vessel goes along in the coelom from the anterior to posterior. Furthermore, on the heart side, a line of white nephridia arrange along the skin. You can also see white muscle underneath the skin and also beside the chaetae.
http://www.beachwatchers.wsu.edu/ezidweb/animals/Abarenicolapacifica2.htm