Of the chromatophore variations throughout the animal kingdom, those within the class Cephalopoda are unique in that they are not controlled via the endocrine system, but rather exclusively by direct innervation. These chromatophores include pigment granules contained within a sac, called a cytoelastic sacculus, surrounded by radial muscles. When contracted these radial muscles expand the sacculus, which provides the epidermis with color characteristic of the pigment. Being covered by these chromatophores, cephalopods are capable of remarkable feats of camouflage.
Fig. 1. Diagram showing the anatomy of a Loligo opalescens chromatophore organ (Cloney and Florey, 1968).
The particular species in discussion here is Octopus rubescens, a local species of the Pacific Northwest. The O. rubescens was unintentionally retrieved while collecting a dead bivalve shell on a dive in the San Juan Islands. The specimen had made its lair out of the shell, and went unnoticed until it was brought back to the lab. The specimen died shortly thereafter.
In the process of dissecting the mantle of the specimen, respiratory activity was still evident. Ethanol was added to ensure death. While observing the epidermis under a dissecting scope, the reduced chromatophores began to show activity.
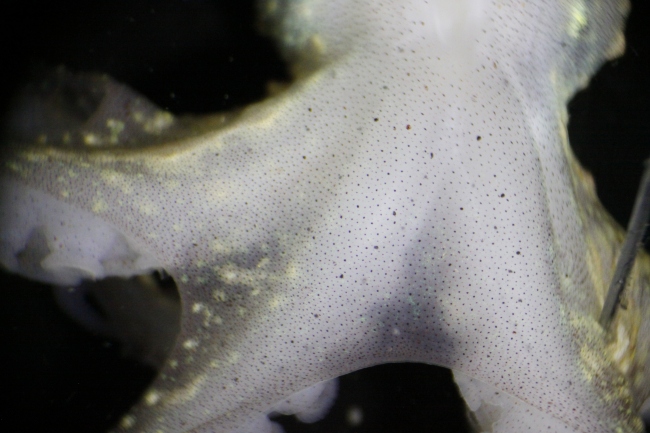
Fig. 2. Posterior side of rubescens during dissection of the mantle, prior to chromatophore activity.
When the radial muscles surrounding chromatophores are relaxed, the elasticity of the tissue retracts the sacculus (Messenger, 2001). The deceased specimen appeared mostly white to the naked eye (see Fig. 2). It was unexpected to find that individual chromatophores, when observing the epidermis at this magnification, were expanding—the radial muscles around them were contracting. As the solution gradually increased in temperature, this activity increased in frequency and magnitude. Eventually, the posterior side of the specimen was ablaze with changing colors visible to the naked eye.
Video 1. 19:26, solution temperature 12.5°C
Video 2. Magnified. 19:32, solution temperature 12.5°C.
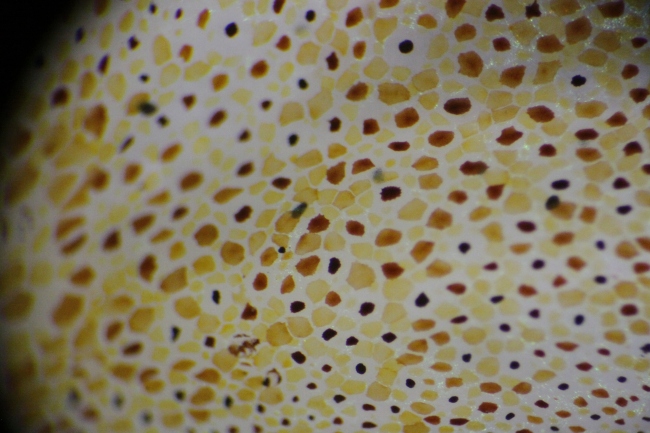
Pigment within the sacculus generally falls toward higher wavelengths of the visible spectrum. Yellow, orange, red, and brown characterized the range found on the O. rubescens (see Fig. 3). These pigments are typically derivatives of tryptophan (Messenger 2001). The activity was visible in waves, and seemed to involve one chromatophore color at a time. Considerable overlap between individual chromatophores was observed when contracted, which, when seen from a distance, could allow further ranges of color. This, along with light reflecting iridophores and the ability of the octopus to contort its epidermis to varying textures, probably aids the octopus in camouflage.
Fig. 3. Magnified epidermis of O. rubescens as chromatophore activity escalated.
The specimen was cooled via refrigeration and addition of seawater to the solution. This was done to minimize time between observations so as to minimize natural degeneration as a factor for reduced activity. At a solution temperature of 7.5 °C, 36 minutes after start of cooling, the specimen was again observed. The chromatophore activity had perceptibly reduced. This observation supports that this chromatophore activity increases with higher temperatures.
Video 3. 20:13, solution temperature 7.5°C.
This activity was known by physiologists as “Wandernwolken” (“wandering clouds”) in the nineteenth century. Messenger explains this phenomenon as correlated with the death of the innervating cells while the surrounding muscles endure, and waves of color as was observed here could be induced via anaesthetization with ethanol. These waves tend to propagate through “classes” of chromatophores, such as similar color or age. This activity lends support to a form of network link between chromatophores. (Messenger, 2001).
Dominic Sivitilli
University of Washington
References:
Cloney, R. A. & Florey, E. (1968). Ultrastructure of cephalopod chromatophore organs. Zeitschrift für Zellforschung 89, 250-280.
Messenger, J. B. (2001), Cephalopod chromatophores: neurobiology and natural history. Biological Reviews, 76: 473–528. doi: 10.1017/S1464793101005772